A surprising new study of the Rembrandt painting The Night Watch revealed the presence of arsenic sulfide minerals.

Public DomainResearchers were curious about this famous Rembrandt painting and decided to try and see how the artist had created it.
Some of Rembrandt’s paintings are so beautiful that they seem to glow. And now, researchers have an idea why. A detailed study of the artist’s work The Night Watch has revealed the presence of arsenic.
Though Rembrandt was not the only artist of his day to use arsenic in his work, he used it in a unique way, which gave his paintings a special sheen.
The Presence Of Arsenic In Rembrandt’s Night Watch Painting
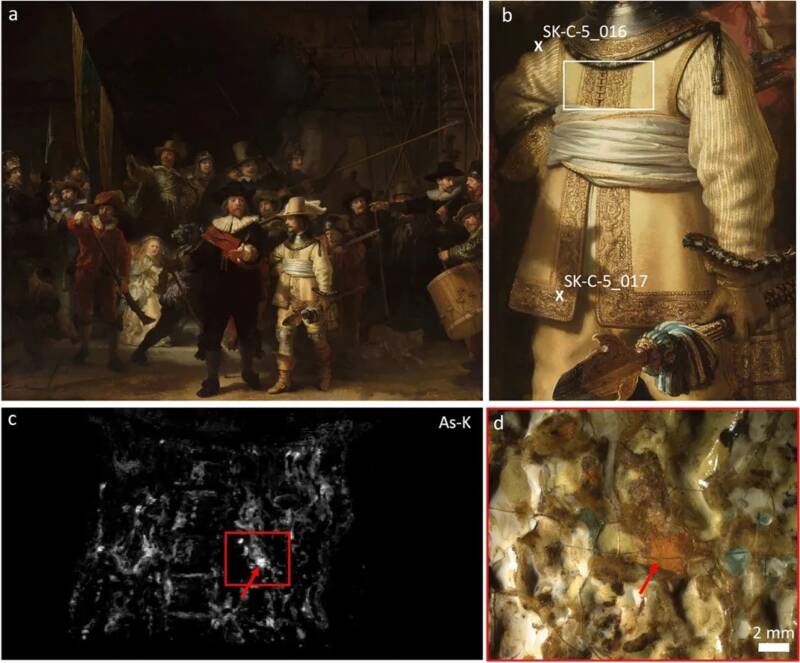
According to a new study published in the journal Heritage Science, scientists had the opportunity to examine Rembrandt’s The Night Watch — one of his most famous works — during “Operation Night Watch,” a study of the artwork conducted by Rijksmuseum in Amsterdam.
The 1642 work of art, which stands 12 feet tall and more than 14 feet long, depicts Amsterdam’s 17th-century guardsmen. It’s a fascinating scene, but researchers were especially intrigued by the golden sheen of the lieutenant figure, Willem van Ruytenburch, who is dressed in gold.
At first, the researchers suspected that Rembrandt had achieved the sheen using orpiment, a “gold pigment” with a bright, yellow tone that was often used to imitate gold. Instead, using high-tech and noninvasive techniques like X-ray scans, they identified “arsenic sulfide pigments” in the “doublet sleeves and embroidered buff coat” worn by van Ruytenburch.
De Keyser et al. via Heritage ScienceResearchers were especially interested in the golden sheen of the lieutenant’s jacket.
Specifically, the researchers found traces of pararealgar and semi-amorphous pararealgar, both arsenic sulfide minerals. They believe that Rembrandt mixed these with other pigments in order to achieve the unique glow of Ruytenburch’s gold clothing.
“Together with the condition of the particles in the paint cross sections, this brings us to the conclusion that Rembrandt intentionally used pararealgar and semi-amorphous pararealgar, together with lead–tin yellow and vermilion, to create an orange paint,” the researchers explained.
This process was not unknown among Dutch artists at the time, but Rembrandt used it in a different way in The Night Watch.
How Rembrandt Used Arsenic In His Paintings
Rembrandt, born Rembrandt Harmenszoon van Rijn in 1606 in Leiden, Netherlands, was the youngest of a large family. He naturally drifted toward painting and art, and studied under some of the most renowned painters of the day. But Rembrandt eventually developed his own technique

Public DomainA self portrait of Rembrandt, the renowned Dutch artist.
According to Artnet, other Dutch artists used arsenic in their still-life paintings, but in slightly different ways. They employed the pigment for scenes that included fruits and flowers; Rembrandt, on the other hand, used it in portraits, as seen in the Night Watch painting.
But the very use of such pigments also tells a story of how arsenic was employed in the 17th century. According to the study, it suggests that it was more widely used by artists during the Dutch Golden Age than was previously known.
“A comprehensive review of historical sources gives insight into the types of artificial arsenic sulfides that were available and suggests that a broader range of arsenic pigments could have been available in Amsterdam in the seventeenth century than previously thought,” the study explains.
As such, the study goes to show that even paintings that are hundreds of years old can reveal new secrets.
After reading about the new study that suggests Rembrandt used arsenic in his paintings, discover the stories of seven surrealists and their most iconic paintings. Or, go inside the life and death of Bob Ross, the beloved artist behind “The Joy of Painting.”





